A team of researchers has succeeded in creating mini insulin-producing organs that can be implanted into a diabetic animal to maintain glucose levels, progress towards what they consider the future of regenerative medicine.
The cells the researchers found are best at producing insulin when reprogrammed are pylotic cells — cells from the lower region of the stomach, called the “pylorus region.”
They think that these cells work best because they are naturally very similar to the pancreatic beta cells that normally carry out this function. What they do better than other cells is respond to high glucose levels by producing insulin to normalize blood sugar levels.
What the researchers first did with their mouse test subjects and what they think could be done for people are two different things.
With mice, the researchers initially reprogrammed cells in their stomachs with conversion genes to become beta cells, and then they destroyed the mice’s pancreatic beta cells, forcing their bodies to rely solely on the artificially created ones. While control mice died within eight weeks, mice possessing the reprogrammed cells lived as long as they were tracked (up to six months).
The researchers also found that pyloric cells had the advantage of naturally renewing themselves — when the researchers destroyed the cells they had created, new ones grew and produced insulin.
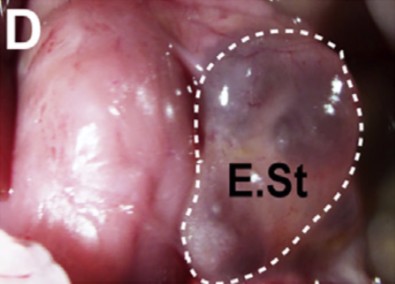
This transgenic experiment would not be used as treatment for diabetes in people, however. Instead, the researchers set about to try something new: they grew tiny stomachs to produce insulin.
They took pyloric tissue out of mice, reprogrammed it to express beta-cell functions, grew the cells in the form of a tiny ball of insulin-producing “stomach,” and put the ball back into the mice. When they destroyed these mice’s pancreatic cells, the engineered organ implants compensated, maintaining normal levels of glucose in five of 22 test animals.
Senior author Dr. Qiao Zhou of the Harvard University Department of Stem Cell and Regenerative Biology explained how they will bridge the gap from the current study to an application for people.
“We are working on two approaches to move this forward toward therapeutics,” Zhou told The Speaker. “One approach is to create engineered human stomach mini-organs from human iPS cells (induced pluripotent stem cells made from the fibroblasts of individual patients) that can produce insulin in culture, followed by transplantation. The other approach is to culture human stomach stem cells from patient biopsy samples, reprogram them into beta-cells in culture, and then transplant them back to the same person. We are making progress on both fronts.”
He also noted the promise offered by engineered therapeutic organs in general.
“The regenerative medicine field has been moving towards a very exciting future of making and engineering entire organs with a complex assembly of different cell types. It is still early but with enormous potential. These organs could replace or supplement the normal function of organs in our body that are failing due to disease or aging. Genetic and bioengineering could be further applied to endow the organs with new function. I believe this is very much the future of regenerative medicine.”
The researchers said they were excited about their success. Replacing insulin-producing pancreatic cells is something science has been trying to do for decades.
“The most surprising part of the study for me is that there are cells residing in your stomach that share surprising features with pancreatic beta-cells,” said Dr. Zhou. “They do not naturally make insulin, but I believe therapeutic methods can be found to “tickle” them to do so. If successfully, it will provide an new approach to treat diabetes.”
Images: The report
Report: Ariyachet et al.: “Reprogrammed stomach tissue as a renewable source of functional beta-cells for blood glucose regulation,” published in Cell.
Link to report