Contrary to intuition, adding pockets of water to solids can actually make them stronger. This finding, the result of research by Yale scientists, offers “a new knob to turn” for engineers, the researchers say. Engineers will be able to add exciting new properties to composite materials–such as electromagnetism–by embedding droplets of liquid, and, on a purely scientific level, the research provides valuable insight into the nature of the material properties at small and large scales–how the relative strengths of a material at one size can be opposite to that at another size.
“This is a great example of how different types of physics emerge at different scales,” Dr. Eric Dufresne, associate professor of mechanical engineering and materials science at Yale and principle investigator of the study, told The Speaker. “Shrinking the scale of an object can really change how it behaves.”

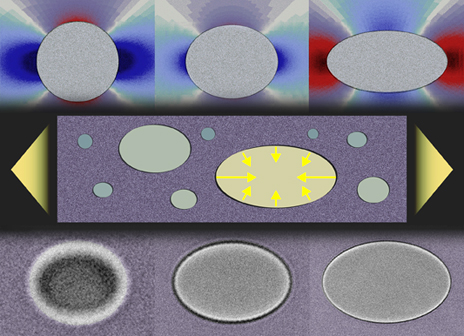
Usually, replacing parts of a solid with liquid–generally considered a soft material–makes the material weaker, but this is not always the case. The researchers strengthened solids with liquids by the virtue of the surface tension of liquid droplets.
“Surface tension is a force that tries to reduce the surface area of a material,” Dufresne told us. “It is familiar in fluids–it’s the force that pulls water into a sponge, makes wet hair clump together and lets insects walk on water. Solids have surface tension too, but usually the ‘elastic force’ of the solid is so strong that surface tension doesn’t have much of an effect. The ‘elastic force’ of a solid is what makes a solid spring back to its originial shape after you stop pushing on it.
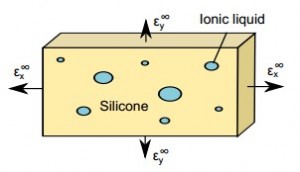
Because the tendency of a liquid is to have as small a surface as possible, embedding small drops of liquid–about a micron in diameter–strengthen solids because the surface tension of the water provides stiffness to the composite.
Dufresne commented on what would be, in his words, “a new knob to turn” for engineers, who can achieve greater control over the properties of composite materials by including fluids.
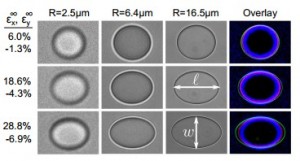
“As the solid gets stiffer, the liquid droplets need to be smaller in order to have this stiffening or cloaking effect. By embedding the solid with droplets of different materials, one can give it new electrical, optical or mechanical properties.
“On the simple scale, they could lower the cost be replacing expensive polymers with simple liquids. More excitingly, embedded droplets could provide an electromagnetic handle to actuate structures.”
In the recent research, the team embedded the small drops of liquid into silicone and then stretched the silicone. Silicone embedded with large drops of water deformed easily–the material was weakened by the liquid. Silicone with small droplets resisted deformation–the material was strengthened.

The team found that a composite up to 30 percent stronger than pure silicone could be created by embedding a large amount of small liquid droplets.
Dufresne explained how the current work came about.
“A few years back, we discovered, on accident, some surprises on how liquid droplets sit on top of solid surfaces,” said Dufresne. “In the course of that work, we realized people needed to pay attention to solid surface tension. Since then, we have been looking for other examples where solid surface tension might be an important and neglected component of the behaviour of materials. These experiments were inspired by ongoing efforts in ‘metamaterials’ where engineers tune the microstructure of a material to give it new properties.”
“It turns out that the importance of surface tension is inversely proportional to the size,” Dufresne said of the study. “So what’s just a negligible force for big things becomes a strong force for very small things–which in turn can strongly affect the material as a whole.”
The report, “Stiffening solids with liquid inclusions,” was completed by Drs. W. Style, Rostislav Boltyanskiy, Benjamin Allen, Katharine E.Jensen, Henry P. Foote, John S. Wettlaufer, and Eric R. Dufresne, and was publishe in December’s Nature Physics.
Photos: all belong to the work of the Yale team





















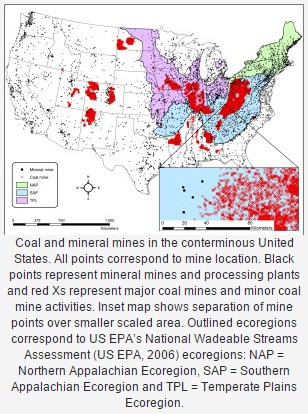 We found that tolerant species decreased in abundance with increased mine density in the watershed.
We found that tolerant species decreased in abundance with increased mine density in the watershed.




 account the moral domains of risk taking as well.”
account the moral domains of risk taking as well.”

